Avogadro Number Calculations II
How Many Atoms or Molecules?
Problems 1 - 10
Problem #1: How many atoms of chlorine are in 16.50 g of iron(III) chloride?
- To go back to the amount of our substance in moles when we have the number of molecules/atoms, all we need to do is divide that value by Avogadro’s Number. 4.5 x 10 23 molecules O 2 ÷ (6.022 x 10 23 molecules / mol) = 0.75 mol O 2.
- Avogadro's number is the number of items in one mole. The number is experimentally determined based on measuring the number of atoms in precisely 12 grams of the carbon-12 isotope, giving a value of approximately 6.022 x 10 23.
- The number 6.022 X 10 23 was given the name Avogadro’s Number by Jean Perrin in 1901. In 1902 Ostwald proposed the term “ mole ” as another way to express Avogadro’s Number. So a mole of carbon ( C ) would have a mass of 12.0107 g and contain 6.022 X 10 23 atoms of carbon.
- Since 90.05 g is equal to 5 moles, we just need to divide the mass by the number of moles. Molar mass H 2 O =!' $ & $ ’ = 18.01 g/mol Correct answer: 18.01 g/mol 7. How many moles are in 45.8 mg of Sr 3 (PO 4) 2?
Example Exercise 9.1 Atomic Mass and Avogadro’s Number. The atomic mass of each element is listed below the symbol of the element in the periodic table: Cu = 63.55 amu, Hg = 200.59 amu, S = 32.07 amu, and He = 4.00 amu. The mass of Avogadro’s number of atoms is the atomic mass expressed in grams. Therefore, 6.02.
Solution:
1) Determine moles of FeCl3:
16.50 g / 162.204 g mol¯1 = 0.101723755 mol
2) Determine how many formula units of iron(III) chloride are in 0.1017 mol:
0.101723755 mol x 6.022 x 1023 = 6.1258 x 1022 formula units
3) Determine number of Cl atoms in 6.1258 x 1022 formula units of FeCl3:
6.1258 x 1022 x 3 = 1.838 x 1023 atoms (to 4 sig fig)
4) Set up using dimensional analysis:
| 1 mol | 6.022 x 1023 | 3 Cl atoms | ||||
| 16.50 g x | –––––––– | x | –––––––––– | x | ––––––––– | = 1.838 x 1023 chlorine atoms |
| 162.204 g | 1 mol | 1 FeCl3 |
Problem #2: How much does 100 million atoms of gold weigh?
Solution:
1) Determine moles of gold in 1.00 x 108 (we'll assume three sig figs in the 100 million):
1.00 x 108 atoms divided by 6.022 x 1023 atoms/mol = 1.660578 x 10¯16 mol
2) Determine grams in 1.66 x 10¯16 mol of gold:
1.660578 x 10¯16 mol times 196.97 g/mol = 3.27 x 10¯14 g (to three sig fig)
Problem #3: 18.0 g of (NH4)2CO3 is present. (a) How many oxygen atoms are present? (b) How many hydrogen atoms are present? (c) How many total atoms are present?
Solution:
1) Convert mass to moles:
18.0 g / 96.0852 g/mol = 0.18733374 mol
2) Determine how many formula units of ammonium carbonate are present:
(0.18733374 mol) (6.022 x 1023 mol¯1) = 1.128124 x 1023 formula units
3) Determine oxygen atoms present:
(1.128124 x 1023 formula units) (3 O atoms / formula unit) = 3.38 x 1023 O atoms
4) Determine hydrogen atoms present:
(1.128124 x 1023 formula units) (8 H atoms / formula unit) = 9.02 x 1023 H atoms
5) Determine total atoms present:
(1.128124 x 1023 formula units) (14 atoms / formula unit) = 1.58 x 1024 atomsFor the total, simply count up how many atoms are in the formula unit. In this case, there are 14 total atoms in one formula unit of (NH4)2CO3 (two N, eight H, one C and three O).
Problem #4: Suppose we knew that there were 8.170 x 1020 atoms of O in an unknown sample of KMnO4. How many milligrams would the unknown sample weigh?
Solution:
1) Determine how many formula units of KMnO4 there are:
8.170 x 1020 atoms divided by 4 atoms per formula unit2.0425 x 1020 formula units of KMnO4
2) Determine moles of KMnO4:
2.0425 x 1020 formula units divided by 6.022 x 1023 formula units/mol = 3.39173 x 10¯4 mol
3) Determine grams, then milligrams of KMnO4:
3.39173 x 10¯4 mol times 158.032 g/mol = 0.0536 g(0.0536 g) (1000 mg/g) = 53.6 mg
4) Dimensional analysis:
| 1 formula unit | 1 mol | 158.032 | 1000 mg | |||||
| 8.170 x 1020 atoms x | –––––––––––– | x | –––––––––– | x | ––––––– | x | ––––––– | = 53.6 mg |
| 4 atoms | 6.022 x 1023 | 1 mol | 1 g |
Problem #5: A solid sample of cesium sulfate contains 5.780 x 1023 cesium ions. How many grams of cesium sulfate must be present?
Solution:
1) Determine how many formula units of Cs2SO4 must be present:
5.780 x 1023 divided by 2 = 2.890 x 1023This is because there are 2 Cs atoms per one cesium sulfate formula unit.
2) Determine how many moles of Cs2SO4 are present:
2.890 x 1023 divided by 6.022 x 1023 mol¯1= 0.479907 mol
3) Determine grams of cesium sulfate:
0.479907 mol times 361.8735 g/mol = 173.7 g
Problem #6: A sample of C3H8 has 4.64 x 1024 H atoms. (a) How many carbon atoms does this sample contain? (b) What is the total mass of the sample?
Solution:
1) Convert from hydrogen atoms to C3H8 molecules:
4.64 x 1024 H atoms divided by 8 H atoms per C3H8 molecule = 5.80 x 1023 molecules of C3H8
2) C3H8 molecules to carbon atoms:
5.80 x 1023 molecules times 3 C atoms per molecule = 1.74 x 1024<---answer for (a)
3) Moles of C3H8:
5.80 x 1023 molecules divided by 6.022 x 1023 molecules / mole = 0.9631352 mol
4) Mass of C3H8:
0.9631352 mol times 44.0962 g/mol = 42.47 gto three sig figs, this is 42.5 g <---answer for (b)
Problem #7: If a sample of disulfur hexabromide contains 6.99 x 1023 atoms of bromine, what is the mass of the sample?
Solution:
1) We need the moles of bromine:
6.99 x 1023 atoms divided by 6.022 x 1023 atoms/mol = 1.160744 mol or Br atoms
2) Disulfur hexabromide's formula is S2Br6. That means there are 6 moles of Br for every one mole of S2Br6, therefore:
1.160744 mol / 6 = 0.193457 mol of S2Br6 present
3) The molar mass of S2Br6 is 543.554 g/mol:
543.554 g/mol times 0.193457 mol = 105.15 gto three sig figs, 105 g
4) Dimensional analysis:
| 1 mol | 1 mol | 543.554 g | ||||
| 6.99 x 1023 atoms x | –––––––––– | x | ––––––– | x | –––––––– | = 105 g |
| 6.022 x 1023 | 6 mol | 1 mol |
Problem #8: A sample of dinitrogen trioxide contains 0.250 moles of oxygen. How many molecules of the compound are present?
Solution:
1) Calculate moles of N2O3:

0.250 mol O times (1 mol N2O3 / 3 mol O) = 0.083333 mol N2O3
2) Calculate molecules of N2O3:
0.083333 mol N2O3 times (6.022 x 1023 molecules / mol) = 5.02 x 1022 molecules (to three sig figs)
Problem #9: Determine the number of oxygen atoms in 2.30 g of Al2(SO4)3.
Solution:
1) Here are the steps:
(a) 2.30 g divided by molar mass of Al2(SOMole Avogadro's Number Worksheet
4)3 = moles of Al2(SO4)3(b) moles of Al2(SO4)3 times 6.022 x 1023 = formula units of Al2(SO4)3
(c) formula units of Al2(SO4)3 times 12 = oxygen atoms in Al2(SO4)3
2) Let's build that up in dimensional analysis style:
| 1 mol | 6.022 x 1023 | 12 O atoms | ||||
| 2.30 g x | ––––––––– | x | –––––––––– | x | ––––––– | = 4.86 x 1022 oxygen atoms |
| 342.147 g | 1 mol | 1 | ||||
| ↑ step (a) ↑ | ↑ step (b) ↑ | ↑ step (c) ↑ |
Problem #10: How many potassium ions are there in 85.0 g of potassium sulfate (K2SO4)?
Solution:
| 1 mol | 6.022 x 1023 | 2 K atoms | ||||
| 85.0 g x | ––––––– | x | –––––––––– | x | ––––––––––– | = 5.87 x 1023 K atoms |
| 174.26 g | 1 mol | 1 formula unit of K2SO4 |
Problem #11: A solution of ammonia and water contains 2.10 x 1025 water molecules and 8.10 x 1024 ammonia molecules. How many total hydrogen atoms are in this solution?
Solution:
Ammonia's formula is NH3 and water's is H2O. Ammonia has three atoms of H per molecule and water has two atoms of H per molecule.
1) Ammonia's contribution:
8.10 x 1024 times 3 = 2.43 x 1025 H atoms
2) Water's contribution:
2.10 x 1025 times 2 = 4.20 x 1025 H atoms
3) Sum them up:
2.43 x 1025 + 4.20 x 1025 = 6.63 x 1025 H atoms
Problem #12: (a) How many water molecules are there in a 4.080 g sample of solid aluminum sulfate octadecahydrate? (b) How many oxygen atoms are there in the 4.080 g sample?
Solution:
1) The formula (and molar mass) of aluminum sulfate octadecahydrate is:
Al2(SO4)3⋅ 18H2O666.4134 g/mol
2) Convert 4.080 g of Al2(SO4)3⋅ 18H2O to moles:
4.080 g / 666.4134 g/mol = 0.006122326 mol
3) Determine formula units of Al2(SO4)3⋅ 18H2O in the 4.080 g:
(0.006122326 mol) (6.022 x 1023 formula units / mol) = 3.68686 x 1021 formula units
4) Every formula unit has 18 water molecules associated with it:
(3.68686 x 1021 formula units) (18 water molecules per formula unit) = 6.636 x 1022 water molecules (answer to a)
5) In 3.68686 x 1021 formula units of Al2(SO4)3⋅ 18H2O, there are a total of 30 oxygen atoms in each formula unit:
(3.68686 x 1021 formula units) (30 O atoms per formula unit) = 1.106 x 1023 O atoms (answer to b)
Problem #13: Determine how many atoms of hydrogen are in 20.0 grams of ammonium chloride.
Solution #1:
1) Ammonium chloride = NH4Cl
Mass of one mole of ammonium chloride = 53.4916 gMass of 4 moles of H = 4.0316 g
Fraction H in NH4Cl = 4.0316 / 53.4916 = 0.075368843
2) To determine the mass of hydrogen in a specific mass of ammonium chloride, multiply by the fraction of H in NH4Cl.
Mass of H = (20.00 g) (0.075368843) = 1.50737686 g
3) Determine moles of hydrogen:
1.50737686 g / 1.008 g/mol = 1.49541355 mol
4) Determine atoms of hydrogen:
(1.49541355 mol) (6.022 x 1023 mole¯1) = 9.00 x 1023 atoms
Solution #2:
1) Convert grams to moles:
20.0 g / 53.4916 g/mol = 0.3738905 mol
2) Convert moles to number of NH4Cl formula units:
(0.3738905 mol) (6.022 x 1023 formula units / mole) = 2.2515686 x 1023 formula units of NH4Cl
3) There are 4 atoms of hydrogen per formula unit:
(2.2515686 x 1023 formula units) (4 atoms / form. unit) = 9.01 x 1023 atoms (rounded to three sig figs)
Problem #14: How many atoms of mercury are present in 9.70 cubic centimeters of liquid mercury? The density of mercury is 13.55 g/cm3. Answer in units of atoms.
Solution:
1) Determine mass of mercury in 9.70 cm3:
(9.70 cm3) (13.55 g/cm3) = 131.435 g
2) Determine moles of mercury in 131.435 g:
131.435 g / 200.59 g/mol = 0.655242 mol
3) Determine atoms in 0.655242 mol:
(0.655242 mol) (6.022 x 1023 atoms/mol) = 3.94 x 1023 atoms
Problem #15: What is the mass of CH4 molecules if they are made from 15.05 x 1023 atoms?
Solution:
1) In 'x' molecules of methane there are:
'x' atoms of C
'4x' atoms of H
2) From which follows this equation:
x + 4x = 15.05 x 1023x = 3.01 x 1023 atoms of C
3) Since there is 1 atom of C for every 1 molecule of CH4, we have:
3.01 x 1023 molecules of CH4
4) Calculate moles of CH4:
3.01 x 1023 molecules divided by 6.02 x 1023 molecules/mol = 0.500 mole of CH4
5) Calculate mass:
0.500 mol times 16.0426 g/mol = 8.02 g (to three sig figs)
Problem #16: 3.00 L of hydrogen gas at SATP would contain how many atoms of hydrogen?
Solution:

1) SATP stands for Standard Ambient Temperature and Pressure and has the following values:
Temperature = 25.0 °CPressure = 100.0 kPa
Note that these values are different from STP. I found the values for SATP here. Look in the table, it's the sixth one down.
2) Use PV = nRT to determine moles of H2 present:
(100.0 kPa / 101.325 kPa/atm) (3.00 L) = (n) (0.08206 L atm / mol K) (298 K)n = 0.121076 mol
I converted kPa to atm because I have memorized the value for R I used. You can look up the value for R expressed in L kPa per mol K, if you wish.
3) Use Avogadro's Number to determine number of molecules:
(0.121076 mol) (6.022 x 1023 molecules/mol) = 7.2912 x 1022 molecules
4) Determine number of atoms:
(2 atoms/molecule) (7.2912 x 1022 molecules) = 1.46 x 1023 atoms
Bonus Problem: A sample of HNO3 contains twice as many atoms as there are atoms in 6.840 g of Al2(SO4)3. Calculate the mass of the HNO3 in the sample.
Solution:
1) We need to first determine the number of atoms in our sample of aluminum sulfate:
6.840 g / 342.147 g/mol = 0.0199914 mol(0.0199914 mol) (6.022 x 1023 formula units/mol) = 1.203882 x 1022 formula units
(1.203882 x 22 formula units) (17 atoms / formula unit) = 2.0466 x 1023 atoms
2) The above calculation done in dimensional analysis style:
| 1 mole | 6.022 x 1023 form. units | 17 atoms | ||||
| 6.840 g x | ––––––– | x | –––––––––––––––––––– | x | ––––––––– | = 2.0466 x 1023 atoms |
| 342.147 g | 1 mol | 1 form. unit |
3) The sample of nitric acid will contain twice as many atoms:
(2.0466 x 1023 atoms) (2) = 4.0932 x 1023 atoms
4) Determine mass of HNO3, done in dimensional analysis style:
| 1 form. unit | 1 mol | 63.0119 g | ||||
| 4.0932 x 1023 atoms x | –––––––––– | x | –––––––––––––––––––– | x | ––––––– | = 8.566 g |
| 5 atoms | 6.022 x 1023 form. units | 1 mol |
Avogadro Number Calculations II
How Many Atoms or Molecules?
The value I will use for Avogadro's Number is 6.022 x 1023 mol¯1.
Types of problems you might be asked look something like these:
0.450 mole of Fe contains how many atoms? (Example #1)0.200 mole of H2O contains how many molecules? (Example #2)
0.450 gram of Fe contains how many atoms? (Example #3)
0.200 gram of H2O contains how many molecules? (Example #4)
When the word gram replaces mole, you have a related set of problems which requires one more step.
And, two more:
0.200 mole of H2O contains how many atoms?
0.200 gram of H2O contains how many atoms?
When the word gram replaces mole, you have a related set of problems which requires one more step. In addition, the two just above will have even another step, one to determine the number of atoms once you know the number of molecules.
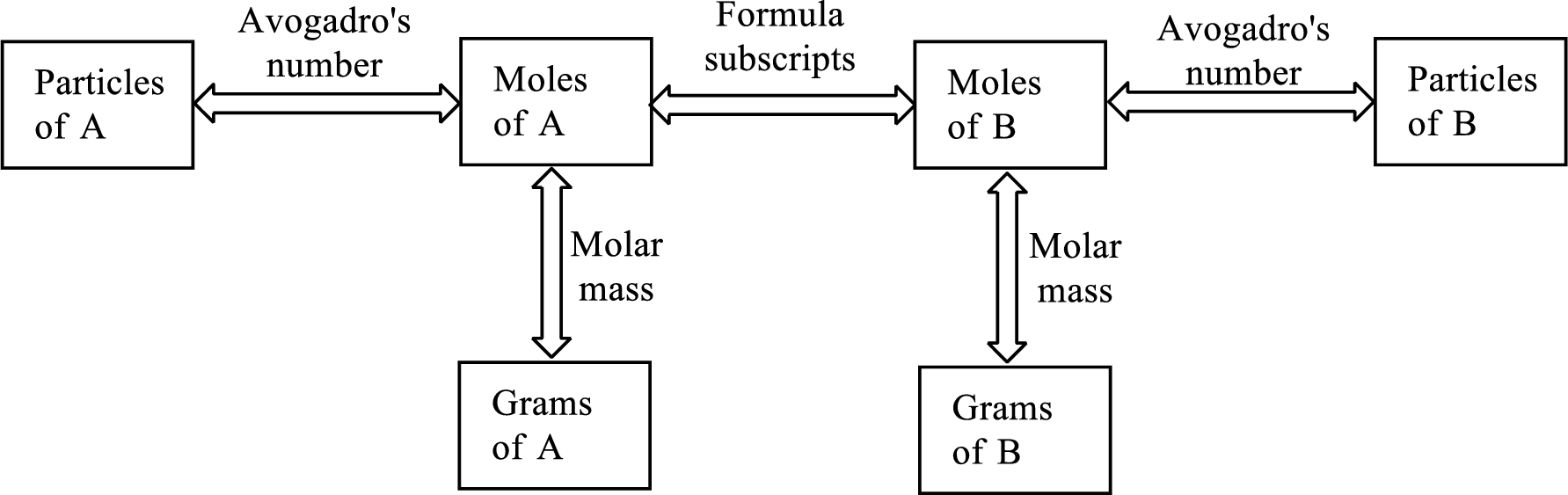
Here is a graphic of the procedure steps:
Pick the box of the data you are given in the problem and follow the steps toward the box containing what you are asked for in the problem.
Example #1: 0.450 mole of Fe contains how many atoms?
Solution:
Start from the box labeled 'Moles of Substance' and move (to the right) to the box labeled 'Number of Atoms or Molecules.' What do you have to do to get there? That's right - multiply by Avogadro's Number.0.450 mol x 6.022 x 1023 mol¯1 = see below for answer
Example #2: 0.200 mole of H2O contains how many molecules?
Solution:
Start at the same box as Example #1.0.200 mol x 6.022 x 1023 mol¯1 = see below for answer
The answers (including units) to Examples #1 and #2
The unit on Avogadro's Number might look a bit weird. It is mol¯1 and you would say 'per mole' out loud. The question then is WHAT per mole?
The answer is that it depends on the problem. In the first example, I used iron, an element. Almost all elements come in the form of individual atoms, so the correct numerator with most elements is 'atoms.' (The exceptions would be the diatomic elements plus P4 and S8.)
So, doing the calculation and rounding off to three sig figs, we get 2.71 x 1023 atoms. Notice 'atoms' never gets written until the end. It is assumed to be there in the case of elements. If you wrote Avogadro's Number with the unit atoms/mol in the problem, you would be correct.
The same type of discussion applies to substances which are molecular in nature, such as water. So the numerator I would use in example #2 is 'molecule' and the answer is 1.20 x 1023 molecules.
Once again, the numerator part of Avogadro's Number depends on what is in the problem. Other possible numerators include 'formula units,' ions, or electrons. These, of course, are all specific to a given problem. When a general word is used, the most common one is 'entities,' as in 6.022 x 1023 entities/mol.
Keep this in mind: the 'atoms' or 'molecules' part of the unit is often omitted and simply understood to be present. However, it will often show up in the answer. Like this:
0.450 mol x 6.022 x 1023 mol¯1 = 2.71 x 1023 atoms
It's not that a mistake was made, it's that the 'atoms' part of atoms per mole was simply assumed to be there.
Example #3: 0.450 gram of Fe contains how many atoms?
Example #4: 0.200 gram of H2O contains how many molecules?
Look at the solution steps in the image above and you'll see we have to go from grams (on the left of the image above) across to the right through moles and then to how many atoms or molecules.
Solution to Example #3:
Step One (grams ---> moles): 0.450 g / 55.85 g/mol = 0.0080573 molStep Two (moles ---> how many): (0.0080573 mol) (6.022 x 1023 atoms/mol) = 4.85 x 1021 atoms
Solution to Example #4:
Step One: 0.200 g / 18.015 g/mol = 0.01110186 molStep Two: (0.01110186 mol) (6.022 x 1023 molecules/mol) = 6.68 x 1021 molecules
Example #5: Calculate the number of molecules in 1.058 mole of H2O
Solution:
(1.058 mol) (6.022 x 1023 mol¯1) = 6.371 x 1023 molecules
Example #6: Calculate the number of atoms in 0.750 mole of Fe
Solution:
(0.750 mol) (6.022 x 1023 mol¯1) = 4.52 x 1023 atoms (to three sig figs)
Example #7: Calculate the number of molecules in 1.058 gram of H2O
Solution:
(1.058 g / 18.015 g/mol) (6.022 x 1023 molecules/mole)Here is the solution set up in dimensional analysis style:
| 1 mol | 6.022 x 1023 | |||
| 1.058 g x | ––––––––– | x | –––––––––– | = 3.537 x 1022 molecules (to four sig figs) |
| 18.015 g | 1 mol | |||
| ↑ grams to moles ↑ | ↑ moles to ↑ molecules | |||
Example #8: Calculate the number of atoms in 0.750 gram of Fe
(0.750 gram divided by 55.85 g/mole) x 6.022 x 1023atoms/mole| 1 mol | 6.022 x 1023 | |||
| 0.750 g x | ––––––––– | x | –––––––––– | = 8.09 x 1021 atoms (to three sig figs) |
| 55.85 g | 1 mol |
Example #9: Which contains more molecules: 10.0 grams of O2 or 50.0 grams of iodine, I2?
Solution:
Basically, this is just two two-step problems in one sentence. Convert each gram value to its mole equivalent. Then, multiply the mole value by Avogadro's Number. Finally, compare these last two values and pick the larger value. That is the one with more molecules.
| 1 mol | 6.022 x 1023 | |||
| 10.0 g x | ––––––––– | x | –––––––––– | = number of O2 molecules |
| 31.998 g | 1 mol |
| 1 mol | 6.022 x 1023 | |||
| 50.0 g x | ––––––––– | x | –––––––––– | = number of I2 molecules |
| 253.8 g | 1 mol |
Example #10: 18.0 g of H2O is present. (a) How many oxygen atoms are present? (b) How many hydrogen atoms are present?
Solution:
1) Convert grams to moles:
18.0 g / 18.0 g/mol = 1.00 mol
2) Convert moles to molecules:
(1.00 mol) (6.02 x 1023 mol¯1) = 6.02 x 1023 molecules
3) Determine number of atoms of oxygen present:
(6.02 x 1023 molecules) (1 O atom / 1 H2O molecule) = 6.02 x 1023 O atoms
4) Determine number of atoms of hydrogen present:
(6.02 x 1023 molecules) (2 H atoms / 1 H2O molecule) = 1.20 x 1024 H atoms (to three sig figs)
Notice that there is an additional step (as seen in step 3 for O and step 4 for H). You multiply the number of molecules times how many of that atom are present in the molecule. In one molecule of H2O, there are 2 atoms of H and 1 atom of O.
Sometimes, you will be asked for the total atoms present in the sample. Do it this way:
(6.02 x 1023 molecules) (3 atoms/molecule) = 1.81 x 1024 atoms (to three sig figs)
The 3 represents the total atoms in one molecule of water: one O atom and two H atoms.
Example #11: Which of the following contains the greatest number of hydrogen atoms?
(a) 1 mol of C6H12O6
(b) 2 mol of (NH4)2CO3
(c) 4 mol of H2O
(d) 5 mol of CH3COOH
Solution:
1) Each mole of molecules contains N number of molecules, where N equals Avogadro's Number. How many molecules are in each answer:
(a) 1 x N = N
(b) 2 x N = 2N
(c) 4 x N = 4N
(d) N x 5 = 5N
2) Each N times the number of hydrogen atoms in a formula equals the total number of hydrogen atoms in the sample:
(a) N x 12 = 12N(b) 2N x 8 = 16N
(c) 4N x 2 = 8N
(d) 5N x 4 = 20N
(d) is the answer.
Example #12: How many oxygen atoms are in 27.2 L of N2O5 at STP?
Solution:
1) Given STP, we can use molar volume:
27.2 L / 22.414 L/mol = 1.21353 mol
2) There are five moles of O atoms in one mole of N2O5:
(1.21353 mol N2O5) (5 mol O / 1 mol N2O5) = 6.06765 mol O
3) Use Avogadro's Number:
(6.06765 mol O) (6.022 x 1023 atoms O / mole O) = 3.65 x 1024 atoms O (to three sig figs)
Example #13: How many carbon atoms are in 0.850 mol of acetaminophen, C8H9NO2?
Solution:
1) There are 8 moles of C in every mole of acetaminophen:
(0.850 mol C8H9NO2) (8 mol C / mol C8H9NO2) = 6.80 mol C
2) Use Avogadro's Number:
(6.80 mol C) (6.022 x 1023 atoms C / mole C) = 4.09 x 1024 atoms C (to three sig figs)
Example #14: How many atoms are in a 0.460 g sample of elemental phosphorus?

Solution:
Phosphorus has the formula P4. (Not P!!)0.460 g / 123.896 g/mol = 0.00371279 mol
(6.022 x 1023 molecules/mol) (0.00371279 mol) = 2.23584 x 1021 molecules of P4
(2.23584 x 1021 molecules) (4 atoms/molecule) = 8.94 x 1021 atoms (to three sig figs)
Set up using dimensional analysis style:
| 1 mol | 6.022 x 1023 molecules | 4 atoms | ||||
| 0.460 g x | –––––––– | x | –––––––––––––––––– | x | ––––––––– | = 8.94 x 1021 atoms |
| 123.896 g | 1 mol | 1 molecule |
Example #15: Which contains the most atoms?
(a) 3.5 molecules of H2O
(b) 3.5 x 1022 molecules of N2
(c) 3.5 moles of CO
(d) 3.5 g of water
Solution:
The correct answer is (c). Now, some discussion about each answer choice.Choice (a): You can't have half of a molecule, so this answer should not be considered. Also, compare it to (b). Since (a) is much less than (b), (a) cannot ever be the answer to the most number of atoms.
Choice (b): this is a viable contender for the correct answer. Since there are two atoms per molecule, we have 7.0 x 1022 atoms. We continue to analyze the answer choices.
The Avogadro Number
Choice (c): Use Avogadro's number (3.5 x 1023 mol¯1) and compare it to choice (b). You should be able to see, even without the 3.5 moles, choice (c) is already larger than choice (b). Especially when you consider that N2 and CO both have 2 atoms per molecule.
Choice (d): 3.5 g of water is significantly less that the 3.5 moles of choice (c). 3.5 / 18.0 equals a bit less that 0.2 moles of water.
Bonus Example: A sample of C3H8 has 2.96 x 1024 H atoms.
(a) How many carbon atoms does the sample contain?
(b) What is the total mass of the sample?
Solution to (a):
1) The ratio between C and H is 3 to 8, so this:
| 3 | y | |
| ––––––– | = | –––––––––––––––– |
| 8 | 2.96 x 1024 H atoms |
2) will tell us the number of carbon atoms present:
y = 1.11 x 1024 carbon atoms
3) By the way, the above ratio and proportion can also be written like this:
3 is to 8 as y is to 2.96 x 1024Be sure you understand that the two different ways to present the ratio and proportion mean the same thing.
Solution to (b) using hydrogen:
1) Determine the moles of C3H8 present.
2.96 x 1024 / 8 = 3.70 x 1023 molecules of C3H8
2) Divide by Avogadro's Number:
3.70 x 1023 / 6.022 x 1023 mol¯1 = 0.614414 mol <--- I'll keep some guard digits
3) Use the molar mass of C3H8:
Grams To Moles Using Avogadro's Numbers
0.614414 mol times 44.0962 g/mol = 27.1 g (to three sig figs)
